THERMAL EXPANSION AND CONTRACTION
Thermal Expansion And Contraction
Solids, liquids and gases generally have increment in size or they expand in all directions when heated. When the solid, liquid or gas is cooled down it decreases in size and contract in all directions. Different substances expand or contract by different amounts for the same temperature change. Generally gases expand the most upon heating as compared to solids and liquids.
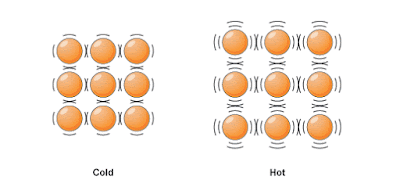 When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
When a substance cool down its molecules slow down and gets closer. This results in contraction of a substance.
 When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
No comments:
Post a Comment