Habitat
A habitat is the natural home of a living thing where it lives and reproduce. Some examples of habitats are a lake, river or spring. A wide variety of microscopic animals is found in a habitat. A place is suitable to be the habitat of an organism if it contain the amount of food needed, shelter from attackers, protection from bad weather and a place for reproducing, nest or hibernation. Everything around an organism that influence its way of life is called its environment. The environment of an organism can be further classified into physical and biotic environment. The physical environment is comprised of physical factors which are non-living such as water, minerals, temperature, light, air and pH value. The biotic environment comprises of other living things which in any way affects the life of the organism. The study of living organisms and their environment is called ecology.
The Physical Environment
The temperature of a place plays an important role in changing the physical environment of that place. It affects the types of organisms which will live there.
Light
All green plants use light energy from sun to make their own food in the process of photosynthesis. Most of the life on Earth depend on plants for food in two ways; directly or indirectly. They also depend on light for surviving. Light also helps living things to see so that they can move from one place to another to find food and detect danger. Some organisms such as earthworm prefer to live in dark. They have special features to live in a dark place. Insects such as fireflies and deep-sea fish produce their own light by mixing chemicals in them.
Temperature
 The heat received from the sun affects the temperature of a place. Most organisms are active at temperatures between 0 degree C and 45 degree C. Temperature also affects the process of reproduction in plants. Some plants will not develop seeds until they are subjected to certain temperatures for a period of time.
The heat received from the sun affects the temperature of a place. Most organisms are active at temperatures between 0 degree C and 45 degree C. Temperature also affects the process of reproduction in plants. Some plants will not develop seeds until they are subjected to certain temperatures for a period of time.
The Biotic Environment

No man is an island. This is not true for man it is also true for other living organisms . Each organism is dependant on other organism by means of feeding, competing with them for food, light, water, air and minerals. The relationships between organisms include
predator - prey relationship, mutualism,commensalism,parasitism.
Thermal Expansion And Contraction
Solids, liquids and gases generally have increment in size or they expand in all directions when heated. When the solid, liquid or gas is cooled down it decreases in size and contract in all directions. Different substances expand or contract by different amounts for the same temperature change. Generally gases expand the most upon heating as compared to solids and liquids.
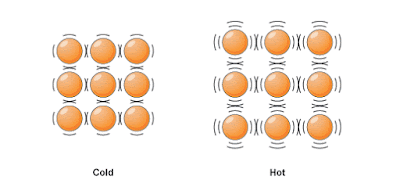 When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
When particles are heated, they start more vibrating and take more place. When a substance is heated, heat energy is absorbed and it makes the molecules to vibrate more quickly. Because of more vibration they start to move apart.
When a substance cool down its molecules slow down and gets closer. This results in contraction of a substance.
Suspensions
A suspension is a mixture in which small particles of solid or liquid are changed into liquid or gas respectively. Example: oil and water. A suspension is mostly cloudy and there is a diverse in the substance. The insoluble particles in a suspension are big enough to prevent light from passing through the suspension. The nature of suspensions are investigated experimentally by doing some laboratory tests. A suspension that contain a large amount of insoluble particles is called a slurry.
Solubility
 A substance which can dissolve in a solvent is said to be a soluble.For example salt is a soluble substance but it has a condition that it can be dissolved in water but not in oil. Solubility is the maximum amount of a solute which can dissolve in a particular amount of solvent at a fixed temperature. Factors of affecting the solubility of a solute in a solvent.
A substance which can dissolve in a solvent is said to be a soluble.For example salt is a soluble substance but it has a condition that it can be dissolved in water but not in oil. Solubility is the maximum amount of a solute which can dissolve in a particular amount of solvent at a fixed temperature. Factors of affecting the solubility of a solute in a solvent.
- the nature of the solute
- the temperature of the solution
- the nature of the solvent
Crystals
Crystals are solids with fixed, regular patterns. Snowflakes, diamonds and table salt are some examples of large crystals. Different types of crystals have different geometrical shape because their atoms are arranged differently. Although we cannot see the atoms present inside a crystal but by looking at the shape of the crystal we get to the arrangement of atoms in it. For instance crystal of sodium chloride is cubic because the ions of sodium and chlorine are arranged in cubes. X-rays can be used to find the atomic structure of different crystals.
 There are seven different crystal systems according to their atomic structure. They are below:
There are seven different crystal systems according to their atomic structure. They are below:
- Cubic System
- Trigonal System
- Tetragonal System
- Hexagonal System
- Rhombic System
- Monoclinic System
- Triclinic System
Applications of crystallisation help in producing silicon wafers for making microchips and solar cells, and in producing refined sugar.
Acids
 A slice of pineapple, a car battery and a bee sting all contain a similar kind of solution called acid. Acids are all around us - the food we eat and in our body also. Malic acid, phosphoric acid, methanoic acid and citric acid are some examples of weak acids. Hydrochloric acid is known to be a strong acid which is produced in our stomach to help in digesting food and for killing bacteria. Nitric acid in another example of strong acid which is used in the manufacture of organic and inorganic nitrates. It is also very corrosive. Jewellers use the fact that acids are corrosive to test the purity of gold. The acid is added and its strength is gradually increased. Pure gold remain unmarked, cheaper metals either stain or corrode.
A slice of pineapple, a car battery and a bee sting all contain a similar kind of solution called acid. Acids are all around us - the food we eat and in our body also. Malic acid, phosphoric acid, methanoic acid and citric acid are some examples of weak acids. Hydrochloric acid is known to be a strong acid which is produced in our stomach to help in digesting food and for killing bacteria. Nitric acid in another example of strong acid which is used in the manufacture of organic and inorganic nitrates. It is also very corrosive. Jewellers use the fact that acids are corrosive to test the purity of gold. The acid is added and its strength is gradually increased. Pure gold remain unmarked, cheaper metals either stain or corrode.
General Properties Of Acids
Most acids, especially the strong ones, are dangerous liquids which can damage our skin also they can react with many materials including metals. So be careful while using them. Whenever by mistake an acid gets into your mouth, quickly spit it and rinse your mouth properly with plenty of water. If any acid is spilt on your parts of body or clothing wash it many times with much water.
All acids have a sour taste.
Acids turn blue litmus paper red.
Aqueous solutions of acids are good conductors of electricity. This is the reason to why sulphuric acid is used in batteries of cars, to produce energy for starting the car.
Dilute acids react with active metals for producing hydrogen gas.
Dilute acids react with carbonates to produce carbon dioxide gas.
Acids react with alkalis to form salts and water.

Refraction Of Light
Light travels at different speeds in different media. When light passes from one transparent medium into another, it may either slow down or travel faster. This change in the speed of light moving through different media causes the light to bend when it hits the surface at an angle to the normal. Refraction of light is the change in direction of light when it moves from one transparent medium to another due to the difference in the speed of light in the two different media.
If light increases its speed when passing from one medium to another, it will bend away from the normal. If the speed of light decreases when travelling from one medium to another, it will bend towards the normal.
Light travels more slowly in glass than in air. When light moves from the air into glass at an angle, one side of the light beam slows down as compared to the other. This change in speed makes the light bend.
Reflection Of Light
When you throw anything against the wall, it bounces back off the wall. Similarly, light travelling in a straight line bounces back or reflects when it strikes the surface of another medium. The amount and direction of the reflected light depends on the surface it strikes.
When a parallel light beam strikes a smooth surface, it will be reflected as a parallel beam. Non-parallel light rays striking a smooth surface will produce either diverging or converging reflected rays which form sharp images. Reflection from a smooth surface is called regular reflection. Mirrors and polished metals have smooth surfaces which produce regular reflections and sharp images.

 Parallel rays of light falling onto a rough surface are scattered in different directions. This type of reflection is called diffused or scattered reflection and no images are formed.
Parallel rays of light falling onto a rough surface are scattered in different directions. This type of reflection is called diffused or scattered reflection and no images are formed.
 The heat received from the sun affects the temperature of a place. Most organisms are active at temperatures between 0 degree C and 45 degree C. Temperature also affects the process of reproduction in plants. Some plants will not develop seeds until they are subjected to certain temperatures for a period of time.
The heat received from the sun affects the temperature of a place. Most organisms are active at temperatures between 0 degree C and 45 degree C. Temperature also affects the process of reproduction in plants. Some plants will not develop seeds until they are subjected to certain temperatures for a period of time.









